Case Report
Atopic Conjunctivitis in Children: Influence of Treatment with Topical Cyclosporin 0.05% in the Quality of Life

Carlos Alberto Sánchez Salguero1* and Álvaro Isidro Sánchez Chacón2
1Head of Pediatric Allergy and Pneumology Section, Professor of Pediatric, School of Medicine. University Hospital Puerto Real, Cádiz, Spain
2Pediatric Department, University Hospital Puerto Real, Cádiz, Spain
*Address for Correspondence: Carlos Alberto Sánchez Salguero, Head of Pediatric Allergy and Pneumology Section, Professor of Pediatric, School of Medicine, University Hospital Puerto Real, Spain, Email: [email protected]
Dates: Submitted: 20 December 2016; Approved: 28 January 2017; Published: 31 January 2017
How to cite this article: Sánchez Salguero CA, Sánchez Chacón ÁI. Atopic Conjunctivitis in Children: Influence of Treatment with Topical Cyclosporin 0.05% in the Quality of Life. Arch Asthma Allergy Immunol. 2017; 1: 001-008. DOI: 10.29328/journal.aaai.1001001
Copyright License: © 2017 Sánchez Salguero CA et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Allergy; Atopic; Conjunctivitis; Children; Cyclosporine 0.05%; Quality of life
ABSTRACT
Introduction: Forty-six per cent children have allergic rhinoconjunctivitis (Allergologica 2005).
Working hypothesis: Ocular topical cyclosporine improves the quality of life for these patients.
Material, methods, design: 2-year prospective study (2015-16), 40 patients with topical corticosteroids without improvement, followed 20 and 20 switched to corticosteroids cyclosporine 0.05%. Interview with Quality of Life Questionnaire in Children with rhinoconjunctivitis (PRQLQ) before and at the end of treatment. Mean age of 10.3 years with 60% male-40% female. Treatments were applied from January to March. There were 15 questions divided into two blocks. Children responded using a card with responses rated from zero (not bothered at all) to 6 (quite upset).
Results: Before the 100% reported that, the itching was very bothersome. In the group of 40 children, 80% showed symptoms of epiphora and 60% showed symptoms of ocular inflammation. 100% complained of significant discomfort in rubbing their eyes, 30% did not like to take medications. Headaches affected 20%. 100% stated that they cannot play normally. 80% showed decreased concentration in class.
Continuing with corticosteroids did not show statistically significant changes.
Patients with cyclosporine improved the results by 3 points, with decreased itching, tearing, swelling, pain, eye rubbing, medication and headaches. In the 2nd questionnaire there was limited variation in the results related to fatigue, malaise, and irritability but with substantially improved balance of sleep, insomnia and concentration at school.
Conclusion: Cyclosporine A is a cyclic polypeptide calcineurin inhibitor developed from the fungus Tolypocladium inflatum. The first dilution was 2% but it is currently used at a dilution of 0.05% and recent publications suggest nanosuspensions. Our study showed improvement in parameters related to symptoms, especially itching and lower improvement of psychological aspects, this achieves a better quality of life for children and more willingness to adhere to the treatment.
INTRODUCTION
Approximately 30% of the population suffers from an allergy, and allergic conjunctivitis affects 40-80% of allergic patients, with an increased prevalence in Western countries [1]. Inflammation of the conjunctiva of the eye is often associated with rhinitis and sometimes asthma. In the pediatric age group <14 years, 46% of patients with allergic phenotype had symptoms of allergic rhino conjunctivitis (Allergologica 2005) [1]. The use of symptomatic medication varies depending on the underlying condition of the patients’ eye: topical and oral antihistamines, topical corticosteroids, stabilizing mast cell membrane, calcineurin inhibitors (cyclosporine A, topical pimecrolimus and tacrolimus).
Clinical forms
There are four clinical forms of ocular allergy [2]:
1. - Seasonal Allergic Conjunctivitis (SAC), the most frequent.
2. - Perennial Allergic Conjunctivitis (PAC), is present throughout the year.
3. - Vernal keratoconjunctivitis (VKC), typical of pediatric patients.
4. - Atopic keratoconjunctivitis (AKC), more related to atopic dermatitis [3].
PATHOPHYSIOLOGY
Ocular allergy is produced by exposing the ocular mucosa to environmental allergens, which after dissolving in the tears, penetrate into the conjunctiva antibody IgE joining on the surface of mast cells [3]. SAC and PAC forms are mediated by IgE. Symptomatology expression relates to the histamine released by activated mast cells, adding the de novo synthesis of mediators: leukotrienes (LT) and prostaglandins (PG).
Histamine and neurotrophins are responsible for burning eye sensations. Inflammation may be worsened when fibroblasts and vascular endothelial cells are activated after mast cell degranulation. At 10-15 min. the maximum effect of these different mediators is evident: histamine, PG and LT. Growth factors are released which attract eosinophils and neutrophils to the site of inflammation. Ag-presenting cells in the conjunctiva are Langerhans Cells which have the Ag to the CD4+T which secrete IL4 (for the synthesis of IgE) and IL5 (growth factor eosinophils).
Cyclosporine A
Cyclosporine A is a cyclic polypeptide calcineurin inhibitor. It was developed by Novartis Lab in the 70’s from cultures of the fungus Tolypocladium inflatum [4]. The therapeutic effects have been reported in many studies. The first publications date from 1986 when it began using dilutions of 2% [5-7], although trials in children were delayed some time [8,9].
The administration form consists of pharmacological drops for topical administration 3 or 4 times daily. The first tests used dilutions of 2% but it has also been administered at dilutions of 1%, 1.25% [10], and is currently at 0.05% concentration [11,12], the most widely used in children. However, recent studies suggest that nanosuspensions cause less eye irritation, with different polymers as delivery vehicles [13].
MATERIAL, METHODS AND DESIGN
This is a prospective study with a sample size of 40 patients previously diagnosed with Vernal Keratoconjunctivitis and who had previously received different treatments without success. They all had at least 3 months using topical steroids to the eye at least 4 days per week and expressed little improvement in symptoms such as itching. Patients were divided into two groups for analysis: 20 patients continued to receive topical corticosteroids (Fluorometholone ophthalmic FML® ophthalmic suspension 0.1%, Allergan Lab) and 20 patients on corticosteroid replaced by cyclosporine A 0.05% (Restasis®, Allergan Lab). Each day, the steroid group was given one drop in each eye every 12 hours, while the cyclosporine group was given one drop in each eye every 12 hours. Both groups maintained this regimen for 3 months. The patients were monitored using ocular pressure controls at the beginning of the treatment, after one month, after two months and at the end of the treatment phase.
Before starting the treatment, the patients were interviewed about their symptoms using the Questionnaire of Quality of Life in Children with rhinoconjunctivitis (PRQLQ) [14] Spanish version provided by Dr. Elizabeth F. Juniper (Department of Clinical Epidemiology and Biostatistics, McMaster University Medical Center, Hamilton, Ontario, Canada) and at the end of the treatment they were re-interviewed using the same questionnaire. For these interviews, issues related to eye disease were evaluated. There was no investigation into issues related to allergic rhinitis. Patients were randomly selected for inclusion in each study group and were recruited during the years 2015 and 2016 with selection based on objective signs (conjunctival hyperemia, giant papillae, papillae hypertrophy, nodules on bulb) and subjective symptoms (tearing, burning, photophobia, warmth, stinging in the eyes) [15]. The average age of the patients overall was 10.3 years (9.1 to 11.5) with a gender distribution of 60% male and 40% female. Of these patients 80% also had rhinitis and 20% also had asthma (assessed by spirometry at baseline and postbroncodilatación).
All subjects underwent allergy testing to beginning with a skin prick test to inhalant allergens (mites, pollens from grasses, trees, weeds, animal dander, mold, cockroaches) and then studied the sensitized positive RAST. The results showed: 100% susceptible to mites (Dermatophagoides pteronissinus or Dermatophagoides Farinae), plus 30% showed positivity to grass pollen (Dactylis, Poa, Festuca, Phleum and Lolium). The papule of Prick-test should be 3x3 mm and RAST study was considered positive if >2.5 IU / ml. Treatments were applied from January to April in order to assess the impact of allergy to mites avoiding interference from grass pollens, which at that time were low. Once the patients had been divided into groups and without them being aware of which treatment would be administered, each patient was subject to a personal interview at their first visit to the hospital. At this interview, results were obtained for the full set of questions within the study. The quality of life questionnaire is based on questions related to nasal symptoms (4 questions), ocular symptoms (4 questions), practical problems (5 questions), other symptoms (6 questions), and activity limitation (4 questions). For our work the questions used were:
1. How much have the itchy eyes bothered you during the last 7 days?
2. How much have watering eyes bothered you during the last 7 days?
3. How much have swollen eyes bothered you during the last 7 days?
4. How much have you been bothered by pain in the eyes for the last 7 days?
5. How much have you been bothered by having to rub your eyes during the last 7 days?
6. How much have you been bothered by having to take medications for your allergies during the last 7 days?
7. How much have you been bothered by headaches during the last 7 days?
8. How much did your allergies affect your play during the last 7 days?
The questions were carried out by an examiner who was a pediatrician in consultation and gave the child a blue card with the choice of responses. The children had to choose between:
6. I was bothered a lot
5. I was very upset
4. I was quite annoyed
3. I was regularly bothered
2. I was bothered a little
1. I was not bothered much
0. I was not bothered at all.
A second set of questions was:
1. How many times have your allergies made you feel tired during the last 7 days?
2. How many times have your allergies made you feel bad in general during last 7 days?
3. How many times have you felt irritable (cranky) because of your allergies during the past 7 days?
4. How many times have your allergies caused you embarrassment during the last 7 days?
5. How many times have your allergies caused difficulty falling asleep during the last 7 days?
6. How many times have your allergies awakened you in the night during the past 7 days?
7. How many times have your allergies caused difficulty in paying attention during the last 7 days?
Children could respond to this second group of questions:
6. Always
5. Almost always
4. Many times
3. Quite a few times
2. Sometimes
1. Almost never
0. Never
RESULTS
To analyze whether there were differences between the responses of patients before and after treatment we used Student’s t with a confidence interval of 95% (results were considered significant at P<0.05).
The statistical results are the following:
First block of questions
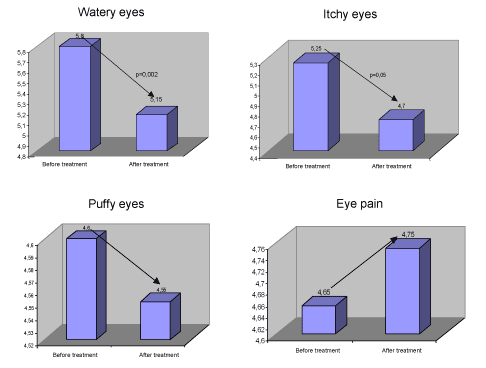
The above analysis shows that the score decreased after treatment significantly (p=0.002). For watering eyes, the tendency was for eyes to water less but the difference found was at the limit of statistical significance (p=0.05).
For puffy eyes there was no statistically significant difference between before and after treatment. For eye pain we found no statistically significant differences.
The patients reported less eye rubbing after the treatment and this difference was statistically significant (p=0.001). The improvement in patients taking less medication for allergy was statistically significant (p=0.01).
There were no statistically significant differences as far as headaches are concerned. There were no statistically significant differences in the discomfort experienced by the patients at play before or after treatment.
Second set of questions
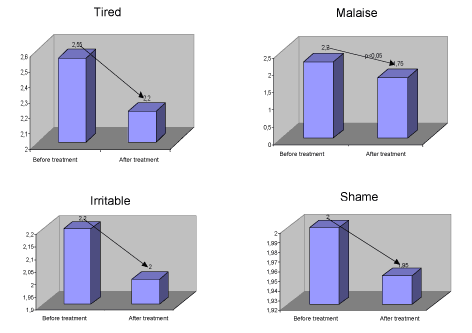
There were no statistically significant differences in terms of fatigue. After treatment patients were feeling less bad in general and this was statistically significant (p<0.05).
| Table 1: Dates about symptoms in the first set of questions and comparative before and after treatment. | |||||||||
| Average before treatment | Average after treatment | T-test for equalityof means t | gl | Cont. | Mean differences | Standard error of the difference | Confidence Interval 95% | ||
| Lower | Higher | ||||||||
| Watery eyes | 5,25 | 4,7 | 2,03 | 38 | 0,05 | 0,55 | 0,27 | 0 | 1,01 |
| 2,03 | 34,82 | 0,05 | 0,55 | 0,27 | 0 | 1,1 | |||
| Itchy eyes | 5,8 | 5,15 | 3,42 | 38 | 0 | 0,65 | 0,19 | 0,27 | 1,04 |
| 3,42 | 29,56 | 0 | 0,65 | 0,19 | 0,26 | 1,04 | |||
| Puffy eyes | 4,6 | 4,55 | 0,19 | 38 | 0,85 | 0,05 | 0,27 | -0,5 | 0,6 |
| 0,19 | 33,52 | 0,85 | 0,05 | 0,27 | -0,5 | 0,6 | |||
| Eye pain | 4,65 | 4,75 | -0,52 | 38 | 0,61 | -0,1 | 0,19 | -0,49 | 0,29 |
| -0,15 | 37,73 | 0,61 | -0,1 | 0,19 | -0,49 | 0,29 | |||
| Medicines | 3,25 | 2,5 | 3,57 | 38 | 0 | 0,75 | 0,21 | 0,33 | 1,18 |
| 3,57 | 34,6 | 0 | 0,75 | 0,21 | 0,32 | 1,18 | |||
| Rub eyes | 5,8 | 5,05 | 3,64 | 38 | 0 | 0,75 | 0,21 | 0,33 | 1,17 |
| 3,64 | 27,85 | 0 | 0,75 | 0,21 | 0,33 | 1,17 | |||
| Headache | 3,65 | 3,05 | 1,54 | 38 | 0,13 | 0,6 | 0,39 | -0,19 | 1,39 |
| 1,54 | 32,52 | 0,13 | 0,6 | 0,39 | -0,19 | 1,39 | |||
| Discomfort to play | 5,15 | 4,95 | 0,67 | 38 | 0,51 | 0,2 | 0,3 | -0,4 | 0,8 |
| 0,67 | 36,14 | 0,51 | 0,2 | 0,3 | -0,4 | 0,8 | |||
There were no statistically significant differences in irritability. There were no statistically significant differences in the number of times they felt embarrassed.
After treatment the patients reported less difficulty sleeping and this was statistically significant (p=0.004). After treatment, patients reported fewer awakenings at night highly statistically significant outcome (p<0.01).
Finally, after treatment, patients reported that allergies less frequently caused them trouble-paying attention. This result was also statistically significant (p<0.01).
DISCUSSION
The result of our study shows a clear improvement in the quality of life for patients treated with cyclosporine A as in other studies [16,17]. In the quality of life questionnaires given to the patients we observed a clear improvement in ocular itching, children reported less time rubbing their eyes to reduce the itching and especially a reduction of the time they remember having felt discomfort from itching. Another point to relate better and closely related to the above is the epiphora. The feeling that the eyes have constant watering is lower and therefore children reported having consumed a smaller amount of tissues.
| Table 2: dates about symptoms in the second set of questions and comparative before and after treatment. | |||||||||
| Average before treatment | Average after treatment | T-test for equalityof means t | gl | Cont. | Mean differences | Standard error of the difference | Confidence Interval 95% Lower |
Higher | |
| Tired | 2,55 | 2,2 | 1,33 | 38 | 0,19 | 0,35 | 0,26 | -0,18 | 0,88 |
| 1,33 | 27,89 | 0,19 | 0,35 | 0,26 | -0,19 | 0,89 | |||
| Malaise | 2,2 | 1,75 | 2,44 | 38 | 0,02 | 0,45 | 0,19 | 0,08 | 0,82 |
| 2,44 | 36,58 | 0,02 | 0,45 | 0,19 | 0,76 | 0,82 | |||
| Irritable | 2,2 | 2 | 1,29 | 38 | 0,21 | 0,2 | 0,16 | -0,12 | 0,52 |
| 1,29 | 34,78 | 0,21 | 0,2 | 0,16 | -0,12 | 0,52 | |||
| Shame | 2 | 1,95 | 0,33 | 38 | 0,75 | 0,05 | 0,15 | -0,26 | 0,36 |
| 0,33 | 37,58 | 0,75 | 0,05 | 0,15 | -0,26 | 0,36 | |||
| Difficulty in sleep | 4,3 | 3,25 | 3,09 | 38 | 0 | 1,05 | 0,34 | 0,36 | 1,74 |
| 3,09 | 36,91 | 0 | 1,05 | 0,34 | 0,36 | 1,74 | |||
| Waking up at night | 4,2 | 2,95 | 4,77 | 38 | 0 | 1,25 | 0,26 | 0,72 | 1,78 |
| 4,77 | 38 | 0 | 1,25 | 0,26 | 0,72 | 1,78 | |||
| Difficulty with attention | 4 | 2,8 | 38 | 0 | 1,2 | 0,27 | 0,66 | 1,74 | |
| 34,4 | 0 | 1,2 | 0,27 | 0,66 | 1,74 | ||||
All these parameters (itchy eyes, rubbing eyes and watering eyes) made children use less medication that alleviate symptoms, and pharmaceutical expenses (as reported by parents) were lower. The main medications, which showed a decrease in consumption, were oral antihistamines.
One of the items that showed improvement in the children was the feeling of malaise, and patients demonstrated improvement of their most bothersome symptoms and especially those, which made them, feel more uncomfortable when with other children. They were in a better mood and more eager to participate in games and activities especially outdoors. This is in contrast to the question about irritability in which there were no statistically significant differences, although we believe it is because the children did not understand the meaning of it (we avoided explaining each item on the questionnaire to obviate external interference or bias in the study).
However, the most striking result from the study is that these children could sleep better-both getting to sleep and staying asleep, especially due to the alleviation of the most troublesome symptoms. This resulted in improved attention at school and improved performance. After the study, we asked some parents to comment on the child´s school performance. The parents reported a significant improvement in school performance for those children treated with cyclosporine.
Topical cyclosporine A 0.05% was used in ocular treatments of 22 patients with steroid-refractory acute keratoconjunctivitis showing some beneficial effects in scores of symptoms and signs [16-19] although some of them used different dilutions to those in our study [20,21].
Finally, the results are in contrast to similar studies [22], although the comparison parameters vary. In most publications, cyclosporine is compared with placebo whilst in our case we compared with corticosteroids.
Our perception of the results is that children show improvement in certain symptoms of their illness and in general have benefitted from an improved quality of life including an improvement in some parameters that allow them to take a more proactive approach to tackle the disease. However, at this stage, we would not strictly recommend cyclosporine A to all patients as a first therapeutic measure as other studies need to be completed which measure changes in the cellularity ocular or inflammatory mediators.
REFERENCES
- Sociedad Española de Alergología e Inmunología Clínica. Alergologica 2005: Factores epidemiológicos, clínicos y socioeconómicos de las enfermedades alérgicas en España. Madrid: Luzán 5 S.A. de Ediciones. 2006.
- Kari O, Saari KM. Updates in the treatment of occular allergies. J Asthma Allergy. 2010; 3: 149-158. Ref.: https://goo.gl/EAAhex
- Stoppel J. Alergia ocular. Rev Med Clin Condes. 2010; 21: 875-882. Ref.: https://goo.gl/ySZI1M
- Shii D, Nakagawa S, Yoshimi M, Katsuta O, Oda T, et al. Symptoms in Late Phase and Delayed-Type Reactions in Allergic Conjunctivitis Models. Biol Pharm Bull. 2010; 33: 1314-1318. Ref.: https://goo.gl/tYLhfq
- BenEzra D, Peer J, Brodsky M, Cohen E. Cyclosporine eye drops for the treatment of severe vernal keratoconjunctivitis. Am J Opthalmol. 1986; 101: 278-282. Ref.: https://goo.gl/xPFJwP
- Secchi AG, Tognon MS, Leonardi A. Topical use of cyclosporine in the treatment of vernal keratoconjunctivitis. Am J Opthalmol. 1990; 110: 641-645. Ref.: https://goo.gl/EYAQIz
- Kaan G, Ozden O. Therapeutic use of topical cyclosporine. Ann Opthalmol. 1993; 25: 182-186. Ref.: https://goo.gl/EXUx6F
- Bleik JH, Tabbara KF. Topical cyclosporine in vernal keratoconjunctivitis. Opthalmology. 1991; 98: 1679-1684. Ref.: https://goo.gl/EesFfJ
- Gupta V, Sahn PK. Topical cyclosporine A in the management of vernal keratoconjunctivitis. Eye (London). 2001; 15: 39-41. Ref.: https://goo.gl/oBDasp
- Spadavechia L, Fanelli P, Tesse R, Brunetti L, Cardinale F, et al. Efficacy of 1.25% and 1% topical cyclosporine in the treatment of severe vernal keratoconjunctivitis in childhood. Pediatr allergy Inmunol. 2006; 17: 527-532. Ref.: https://goo.gl/GxLsVJ
- Duque B, Restrepo CA. Tratamiento de la conjuntivitis alérgica crónica con ciclosporina tópica. Unidad médica CES 1991-1992. Revista CES Mediana. 1992; 6: 2. Ref.: https://goo.gl/Rpn8im
- Ozcan AA, Ersoz TR, Dulger E. Management of severe allergic conjunctivitis with topical cyclosporine A 0.05% eyedrops. Cornea. 2007; 26: 1035-1038. Ref.: https://goo.gl/0zr4G9
- Kim JH, Woo JS, Dug HS, Don HH, Gon CH. Development of a novel ophthalmic cyclosporine A-loaded nanosuspension using top-down media milling methods. Pharmazie. 2011; 66: 491-495. Ref.: https://goo.gl/f9Of72
- Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR. Measuring quality of live in children with rhinoconjunctivitis. J Allergy Clin Inmunol. 1998; 101: 163-170. Ref.: https://goo.gl/4bAHDe
- Tesse R, Spadavecchia L, Fanelli P, Rizzo G, Procoli U, et al. Treatment of several vernal keratoconjunctivitis with 1% topical cyclosporine in an Italian cohort of 197 children. Pediatr Allergy Immunol. 2010; 21: 330-335. Ref.: https://goo.gl/GyNs3P
- Akova YA, Rodriguez A, Foster CS. A randomised trial of topical cyclosporine 0.05% in topical steroid-resistant atopic keratoconjunctivitis. Ocular Immun Inflamm. 1994; 2: 125-144.
- Pucci N, Caputo R, Mori F, De Libero C, Di Grande L, et al. Long-tern safety and efficacy of topical cyclosporine in 156 children with vernal keratoconcjuntivitis. Int J Immunopathol Pharmacol. 2010; 23: 865-871. Ref.: https://goo.gl/Hj1XzT
- Spadavecchia L, Fanelli P, Tesse R, Rizzo G, Procoli U, et al. Prognosis and treatment of vernal keratoconjunctivitis in pediatric age: pilot study on 197 patients. Minerva Pediatr. 2010; 62: 239-244. Ref.: https://goo.gl/scrQdR
- Keklikci U, Sevda Soker I, Yildirim Sakalar B, Kaan Unlu, Selver Ozekinci, et al. Efficacy of topical cyclosporine A 0.05% in conjunctival impression cytology specimens and clinical findings os fevere vernal keratoconjunctivitis in children. Jpn J Ophthalmolol. 2008; 52: 357-362. Ref.: https://goo.gl/9LReSj
- Hingorani M, Moodaley L, Virginia Calder L, Roger Buckley J, Lightman S. A randomised, placebo-controlled trial of topical cyclosporine A in steroid-dependent atopic keratoconcjuntivitis. Ophthalmology. 1998; 105: 1715-1720. Ref.: https://goo.gl/ortAs4
- Tesse R, Fanelli P, Rizzo G, Spadavecchia L, Procoli U, et al. Treatment of severe vernal keratoconjunctivitis with 1% topical cyclosporine in an Italian cohort of 197 children. Pediatr Allergy Immunol. 2010; 21: 330-335. Ref.: https://goo.gl/3CNyCk
- Daniell M, Constantinuo M, Vu H T, Tayleo HR. Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophtalmol. 2006; 90: 461-464. Ref.: https://goo.gl/05P6ts