More Information
Submitted: September 09, 2023 | Approved: July 09, 2024 | Published: July 10, 2024
How to cite this article: Jbeili S, Rima M, Annous AR, Berro AI, Fajloun Z, Karam M. Gentian Violet Modulates Cytokines Levels in Mice Spleen toward an Anti-inflammatory Profile. Arch Asthma Allergy Immunol. 2024; 8: 001-006. Available from: https://doi.org/10.29328/journal.aaai.1001034.
DOI: 10.29328/journal.aaai.1001034
Copyright License: © 2024 Jbeili S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Gentian violet; IFN-γ; TNF-α; IL-1β; IL-10; IL-4; IL-13
Gentian Violet Modulates Cytokines Levels in Mice Spleen toward an Anti-inflammatory Profile
Salam Jbeili1#, Mohamad Rima2#, Abdul Rahman Annous3, Abdo Ibrahim Berro3, Ziad Fajloun4,5 and Marc Karam1*
1Department of Biology, University of Balamand, Kalhat, Al-Kurah, P.O. box 100 Tripoli, Lebanon
2Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), INSERM U964, CNRS U7104, Université de Strasbourg, 67400 Illkirch, France
3Medical Laboratory, Faculty of Health Sciences, University of Balamand, Lebanon
4Laboratory of Applied Biotechnology (LBA3B), Azm Center for Research in Biotechnology and its Applications, Doctoral School for Sciences and Technology, Lebanese University, El Mittein Street, 1300 Tripoli, Lebanon
5Faculty of Sciences 3, Lebanese University, Michel Slayman Tripoli Campus, Ras Maska 1352, Lebanon
#Equal contribution
*Address for Correspondence: Marc Karam, Department of Biology, University of Balamand, Kalhat, Al-Kurah, P.O. box 100 Tripoli, Lebanon, Email: [email protected]
Introduction: Gentian Violet (GV) is a triphenylmethane industrial dye that is known for its antibacterial, antiviral, anti-helminthic, and anti-tumor effects. Although many studies focused on determining the biological and pharmacological applications of GV, its exact effect on the immune response has not been elucidated yet.
Methods: In this study, we investigate the immunomodulatory effects of GV in BALB/c mice after intraperitoneal injection of the dye by assessing cytokines levels in the spleen.
Results: Our data show that GV-treated mice have decreased levels of proinflammatory cytokines (IL-1β and TNF-α) and increased levels of anti-inflammatory cytokines (IL-4) in their spleens. In addition, IFN-γ which can modulate pro-inflammatory cytokine production was upregulated in GV-treated mice.
Conclusion: Together, these findings suggest an anti-inflammatory activity of GV that warrants further studies investigating the potential of GV in immunotherapy.
Gentian Violet (GV) is a triphenylmethane dye used in industry for ink, sanitary products, ceramics, and photo-imaging systems [1,2]. The dye, discovered by Charles Lauth [3], is also used by scientists in different biological applications including bactericidal, antifungal, and anthelminthic activities [4,5]. GV was used in the treatment of skin burns, and dermal and systemic Candidiasis. The molecule was recognized as an antiseptic of wounds and an inhibitor of mold in poultry. It is very beneficial against parasites such as cutaneous Leishmania and Chagas disease in the blood [6]. Gentian Violet was also known for its efficiency against gram-positive organisms [7] as well as Nipah and Hendra viruses [8]. Despite all its benefits, GV is toxic at high doses, and it cannot be managed easily [9-12] as it can be carcinogenic, and cause gastrointestinal side effects and taste alteration in the human body [13,14]. A recent study showed that GV reduces the effect of angiopoietin-2 (ang-2), proinflammatory receptors in angiogenesis [7], which suggests an immunomodulatory effect of the molecule. In agreement with this finding, GV inhibited the proliferation of breast cancer cells by suppressing the activity of the proinflammatory molecule NF-κB [15-17]. Gentian violet was also shown to inhibit reactive oxygen species (ROS), leading to the decline of the inflammatory activity of NF-κB [18,19]. In this study, we investigated the immunomodulatory potential of GV in vivo. We show that GV has anti-inflammatory potential by downregulating proinflammatory cytokines (IL-1β and TNF-α) and upregulating anti-inflammatory cytokines (IL-4) and IFN-γ that modulate pro-inflammatory cytokine production.
Gentian violet
Gentian Violet was supplied from Sigma Aldrich (G2039) in powder form and stored at room temperature. Gentian Violet was dissolved in PBS before the experiment and filtered through 0.2 µm sterile syringe filters.
Mice handling and treatment
Eight to ten-week-old female BALB/c mice procured from the University of Balamand (UOB) animal house were used in this study. Animals were fed a standard diet and kept at 25 °C in 12 12-hour day/night cycles and handled according to the Guide for Care and Use of Laboratory Animals of the UOB Faculty of Sciences. Mice were divided into two groups that were injected intraperitoneally with either Gentian Violet (5 mg/kg or PBS (control). Please note that the dose was selected according to a previous study by our team (unpublished data) in which the LD50 of gentian violet was determined. Each mouse underwent three injections with 48 hours (hrs) of interval between each injection. Mice were sacrificed 24 hrs following the last injection by cervical dislocation. Spleens were removed, weighted, and then cut in half. For histology, spleen parts were reserved in chloroform at -20 °C; while those for ELISA were kept in Eppendorf tubes at -80 °C. All animals were handled and experimental procedures were carried out according to the guidelines of the Institutional Animal Care and Use Committee at the University of Balamand, with strict adherence to the ethical guidelines for the study of experimental pain in conscious animals [20].
Histopathology
Tissue for histopathology was processed as described in [21,22]. Briefly, spleen samples were processed for dehydration, clearing, and impregnation by Leica TP1020 Tissue Processor. Paraffin blocks were prepared using ThermoFisher Histostar Tissue Embedding Station and serial sections of 3 µm thickness were cut using Leica RM2255 Fully Automated Rotary Microtome. Sections were placed on slides with 50 mM ethanol and then immersed in dissolved 0.1% gelatin. Dewaxing was performed by emerging the prepared slides 2 x 5 min in Xylol. Samples were then washed 3 x 2 min with 95% ethanol, then for 2 min with 75% ethanol, then 50% ethanol. Samples were drained for 3 min with water, treated with 0.37% HCL in 70% ethanol to remove hemotoxylin excess, and drained for 2 min with water. The nucleus was stained using ammonia, and the cytoplasm was stained using eosin. Slides were washed 5 x 10 min with 95% ethanol, emerged in Xylol, and mounted with coverslips. Mounted tissue sections were observed under Swift M2250 Series Monocular lab light microscope for structural changes and abnormalities.
Tissue homogenization and protein quantification
Frozen spleen samples were weighted, then homogenized at 4°C in 1 ml RIPA buffer (25 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS pH = 7.6) supplemented with protease inhibitors. The homogenates were incubated on ice for 30 minutes and then centrifuged at 10 000g for 30 minutes at 4 °C. Supernatants were transferred to labeled Eppendorf tubes and the protein concentration of each sample was quantified using a Bio-Rad Protein Assay kit according to the manufacturer’s recommendations. Samples were stored at -80 °C for cytokine measurement.
Cytokine measurement
Quantitative evaluation of cytokines was performed using Enzyme-Linked Immuno-Sorbent Assay (ELISA) Development Kits (Cat. #900-K00, Peprotech) according to the manufacturer’s recommendations. Briefly, 100 μl of prepared supernatants/standards were added into the 96-well plates in duplicates. Plates were read with an ELISA plate reader at 405 nm with 650 nm as the correction wavelength. Concentrations of the cytokines TNF-α, IFN-γ, IL-4, IL-10, IL-1ß, and IL-13 were estimated using standard curves established with the appropriate recombinant cytokines. The results were expressed as pg/mg of total proteins.
Statistical analysis
Differences among groups were analyzed using GraphPad Prism 6.00 software (GraphPad Software Inc., San Diego USA) by one-way analysis of variance (ANOVA). Results were expressed as means ± SEM. p < 0.05 was considered statistically significant.
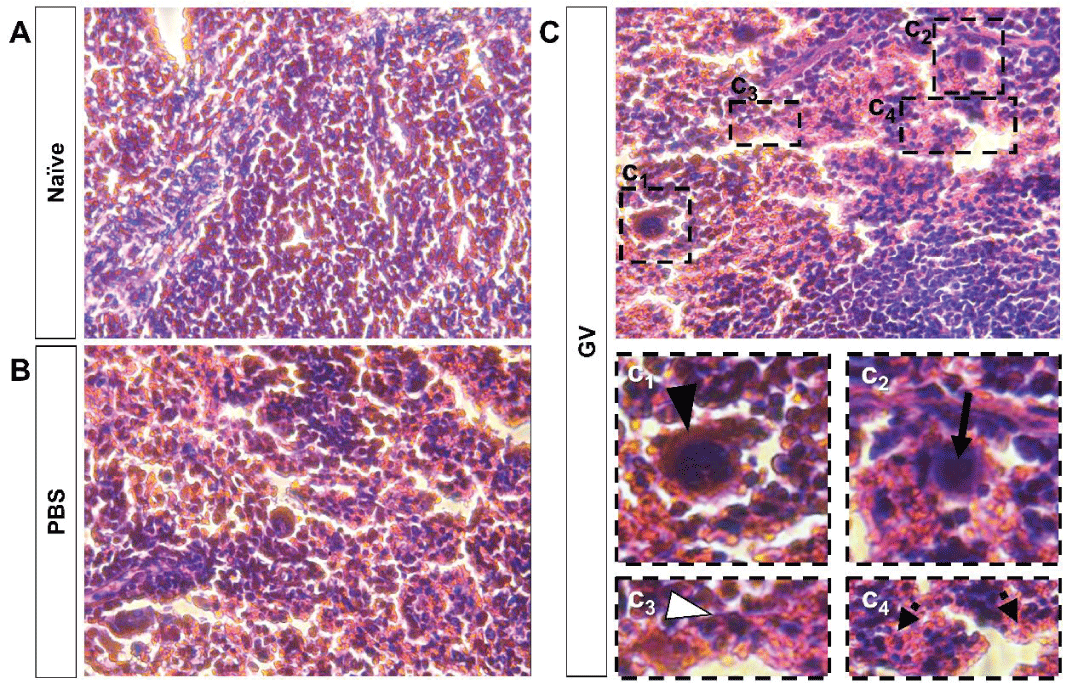
Histological disorganization of GV-injected mice spleen tissue
First, we checked the histology of the mice’s spleen, the site of innate and adaptive immune processes [23], after GV injection. Compared to controls (Figure 1A,B), which did not show any significant changes in spleen tissue histology, we found that spleens from GV-injected mice (Figure 1C) show indeed remarkable histological changes. We reported the presence of megakaryocytes (Figure 1C1) with polynuclear inflammatory infiltration (Figure 1C3) in GV-injected mice spleen tissue. In addition, slight extensions in red pulps (Figure 1C4) and enlarged lymphoid follicles with aggregates of monocyte-like cells (Figure 1C2) were remarked. Together, these findings show that GV injection induces spleen histological disorganization, which suggests an immunomodulatory effect of GV.
Figure 1: Histological changes are observed in the spleen tissue of GV-injected mice. Microscopic observations and histological evaluation of spleen tissue from (A) naïve, (B) PBS-, and (C) GV-injected mice. While (A) naïve and (B) PBS-injected mice don’t show major spleen histological changes, (C) GV-injected mice show megakaryocytes (C1), enlarged lymphoid follicles (C2), polynuclear inflammatory infiltration (C3), and extension in red-pulps (C4) in spleen tissue.
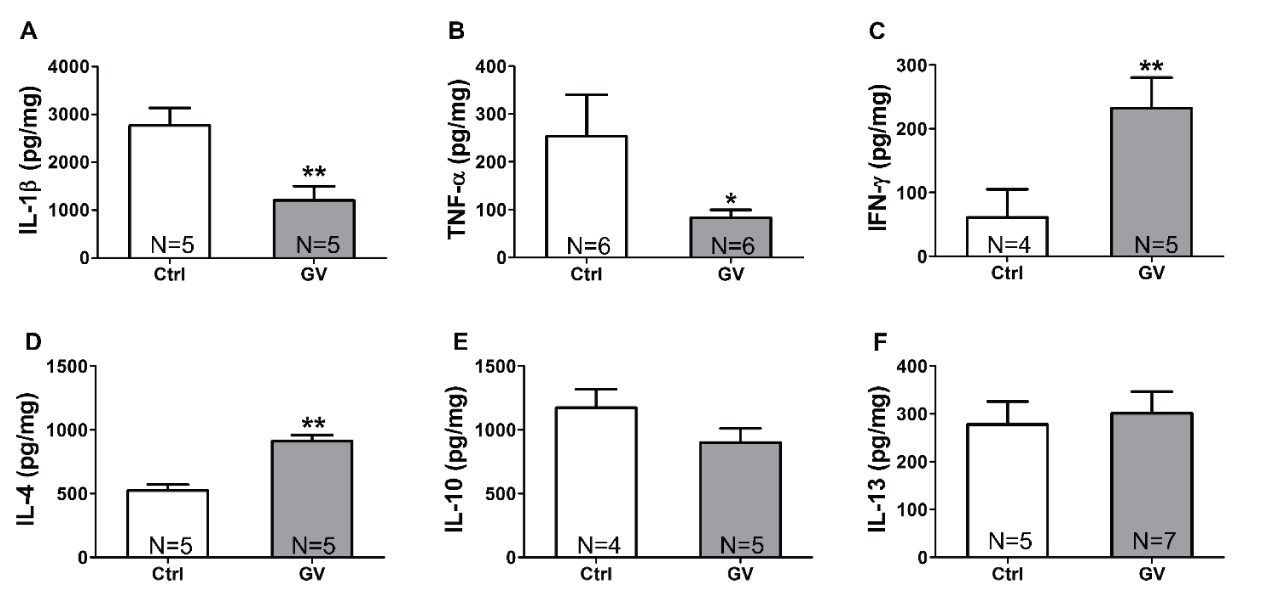
Proinflammatory cytokines levels are decreased in GV-treated mice
Next, we dissected the immunomodulatory effect of GV by investigating changes in pro- and anti-inflammatory cytokine levels in mice spleens after GV intraperitoneal injection. Compared to controls, results show that GV-injected mice show a significant decrease in interleukin-1β (IL-1β) levels, a key mediator of the inflammatory response [24] (Figure 2A). Tumor necrosis factor alpha (TNF-α) levels showed also a significant decrease in GV-injected mice (Figure 2B). TNF-α is a multifunctional cytokine secreted primarily by macrophages, natural killer (NK) cells, and lymphocytes; therefore, holds diverse proinflammatory actions [25]. Given that both downregulated cytokines (IL-1β and TNF-α) are key mediators of pro-inflammatory response, these results suggest that GV negatively affects proinflammatory cytokines levels.
IFN-γ concentration is upregulated upon GV injection
GV-treated mice showed increased interferon-gamma (IFN-γ) levels that were ~ 4.5-fold higher in GV-injected than control mice (Figure 2C). Interestingly, IFN-γ has a dual role in inflammation. IFN-γ is not only associated with the pathogenesis of chronic inflammation, but it can also induce anti-inflammatory molecules and modulate pro-inflammatory cytokine production [26]. Taken with proinflammatory cytokines decrease (IL-1β and TNF-α), our findings suggest that upregulation of IFN-γ is more likely in favor of regulating pro-inflammatory cytokine levels and promoting anti-inflammatory cytokines secretion.
GV increases anti-inflammatory cytokines levels in the spleen of mice
To check whether GV immunomodulatory effect affects anti-inflammatory cytokines, interleukin-4 (IL-4), interleukin-10 (IL-10), and interleukin (IL-13) levels were quantified in GV-treated mice. Our results show a significant increase (~ 2-fold, p < 0.01) in IL-4 concentration (Figure 2D); however, IL-10 and IL-13 levels remain unchanged between GV-injected and control mice (Figure 2E,F). These findings show that GV indeed induces an anti-inflammatory response and selectively increases IL-4, but not IL-10 nor IL-13 levels.
Figure 2: GV modulates cytokines levels in the spleen. Levels of (A) IL-1β, (B) TNF-α, (C) IFN-γ, (D) IL-4, (E) IL-10, and (F) IL-13 were measured using an enzyme-linked immunosorbent assay kit (ELISA). * p < 0.05, ** p < 0.01. IL-1β: Interleukin-1β, TNF-α: Tumor necrosis factor alpha, IFN-γ: Interferon gamma, IL-4: Interleukin-4, IL-10: Interleukin-10, IL-13:
Immunomodulation refers to the regulation of the immune response by suppressing or enhancing its components for therapeutic purposes [27-29]. Since cytokines are key components of immune response, altering released cytokines concentration will have immediate effects on the immune response. This strategy, termed immunotherapy, is used for the treatment of infections, autoimmune diseases, and cancer, and relies on chemical and biological molecules developed by pharmaceutical companies [30]. Among chemical compounds, triphenylmethane dyes have shown anti-inflammatory activities [31].
Gentian Violet (GV) is a triphenylmethane industrial dye that is known for its antibacterial, antiviral, anti-helminthic, and anti-tumor effects [4,5]. This work constitutes the first in vivo study highlighting the immunomodulatory effect of gentian violet. Herein, we show that gentian violet modulates cytokines levels in mice’s spleen. While pro-inflammatory cytokines (IL-1β, TNF-α) levels are decreased, anti-inflammatory cytokine IL-4, and IFN-γ, an inducer of anti-inflammatory molecules and modulator of pro-inflammatory cytokine production, are upregulated. In addition, observed changes in GV-treated mice spleen histology that are characterized by the presence of inflammation, infiltration, and hyperplastic lymphoid follicles emphasize the immunomodulatory role of gentian violet. This activity could be of interest in studies aiming to develop cancer treatment strategies. As such, studies on animal models described cytokines’ roles in the pathogenesis of angiogenesis. IL-1β and TNF-α are potent pro-angiogenic cytokines, while IL-4 are anti-angiogenic cytokines [32,33]. The ability of gentian violet to increase IL-4 and decrease IL-1β and TNF-α levels deserves further studies investigating the anti-angiogenic potential of gentian violet in cancer treatment. In agreement with this hypothesis, IL-4 can exhibit an anti-tumor activity, as it is involved in promoting immune response against tumor models including renal cancer, colorectal cancer, spontaneous adenocarcinoma, colon carcinoma, fibrosarcoma and melanoma [34,35].
Even though IL-13 shares many biological activities with IL-4, the insensitivity of IL-13 levels to gentian violet treatment could be explained by the different T-cell subsets that produce IL-13, which may not be affected by gentian violet treatment. Alternatively, since IL-13 secretion requires CD4+ T cell differentiation into Th2 cells in the presence of IL-4 [36-38], IL-13 levels may increase later as a consequence of IL-4 upregulation. Contrary to Humans, where IL-4 inhibits IFN-γ secretion [39,40], in mice, IL-4 is known to enhance IFN-γ secretion in response to a variety of stimuli [41]. These findings are in agreement with ours showing an increase in IFN-γ levels in GV-treated mice. On the other hand, IL-4, IL-10, and IL-13 were previously shown to inhibit the production of IL-1β and tumor necrosis factor-α [42], which is comparable to our findings showing an increase in IL-4 along with a decrease in IL-1β and TNF-α. These findings suggest that IL-1β and TNF-α levels could be mediated by an IL-4 level increase. It is known that IL-4 plays a crucial role in shaping the nature of immune responses [43] and that IL-4 production is triggered in response to receptor activation by TH2-type CD4+ T cells, basophils, and mast cells [44]. Since gentian violet significantly upregulates IL-4 levels, it is likely to suggest that this dye can activate IL-4-dependant adaptive immune system represented by naïve CD4+ T cells differentiation into Th2 cells [36]. Our findings highlight the importance of depicting the mechanism by which gentian violet triggers IL-4 upregulation and its possible clinical implications either in reducing type 1 immune response (represented by Th1 cells and ILC1) or in enhancing type 2 immune response (represented by Th2 and innate lymphoid cells 2 – ILC2) highly associated with allergy [45].
In a few words, gentian violet seems to shape the immune response toward an anti-inflammatory profile as shown by the reduction of IL-1β and TNF-α levels (produced mainly by macrophages). On the other hand, we show here that this stain can up-regulate both Th1 and Th2 signature cytokines IFN-γ and IL-4 respectively with no major effect on the levels of IL-10 (produced mainly by Treg cells.
This pilot study paves the road for more advanced studies to unravel the wide variety of biological and medical effects of gentian violet and its mechanism of action. Such studies could rely as a start on the assessment of the effect of gentian violet on reactive oxygen species secretion by the different spleen cells in addition to investigating its polarizing potentials (using FACS) on those cells, especially T helper cells and macrophages. The results of those studies warrant further understanding of gentian violet therapeutic potential.
The authors would like to thank Mr. Michel Zakhem and Mrs. Takla El Khoury for their technical help.
Statement of ethics
The authors certify that all animals were handled and experimental procedures of this work were carried out according to the guidelines of the Institutional Animal Care and Use Committee at the University of Balamand (SEED-SJ001), with strict adherence to the ethical guidelines for the study of experimental pain in conscious animals (Zimmermann, 1983).
Funding sources
This research was funded by the University of Balamand.
Author contributions
Author Contributions: M.K. conceived and designed the experiments; S.J. performed the experiments; A.R.A. and A.I.B. contributed to the histopathological study; S.J., M.R., Z.F., and M.K. interpreted the results; J.F., M.R., Z.F., and M.K. wrote the manuscript.
- Zucca P, Cocco G, Sollai F, Sanjust E. Fungal laccases as tools for biodegradation of industrial dyes. Biocatalysis. 2016;1:82-108. Available from: https://www.degruyter.com/document/doi/10.1515/boca-2015-0007/html
- Thetford D. Triphenylmethane and Related Dyes. In: Kirk-Othmer Encyclopedia of Chemical Technology. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2013.
- Lauth C. On the new aniline dye, Violet de Paris. Laboratory. 1867;1:138-9.
- Maley AM, Arbiser JL. Gentian Violet: a 19th-century drug re‐emerges in the 21st century. Exp Dermatol. 2013;22(12):775–80. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/exd.12257
- Conn HJ. An Investigation of American Stains: Report of Committee on Bacteriological Technic. J Bacteriol. 1922;7(1):127-48. Available from: https://pubmed.ncbi.nlm.nih.gov/16558944/
- de Souza Pietra RC, Rodrigues LF, Teixeira E, Fried L, Lefkove B, Rabello A, et al. Triphenylmethane derivatives have high in vitro and in vivo activity against the main causative agents of cutaneous Leishmaniasis. PLoS One. 2013;8(1):e51864. Available from: https://pubmed.ncbi.nlm.nih.gov/23341885/
- Berrios RL, Arbiser JL. Effectiveness of gentian violet and similar products commonly used to treat pyodermas. Dermatol Clin. 2011;29(1):69-73. Available from: https://pubmed.ncbi.nlm.nih.gov/21095530/
- Aljofan M, Sganga ML, Lo MK, Rootes CL, Porotto M, Meyer AG, et al. Antiviral activity of gliotoxin, gentian violet and brilliant green against Nipah and Hendra virus in vitro. Virol J. 2009 Nov 4;6:187. Available from: https://pubmed.ncbi.nlm.nih.gov/19889218/
- Liu W, Chao Y, Yang X, Bao H, Qian S. Biodecolorization of azo, anthraquinonic and triphenylmethane dyes by white-rot fungi and a laccase-secreting engineered strain. J Ind Microbiol Biotechnol. 2004;31(3):127-32. Available from: https://pubmed.ncbi.nlm.nih.gov/15069603/
- Azmi W, Sani RK, Banerjee UC. Biodegradation of triphenylmethane dyes. Enzyme Microb Technol. 1998; 15;22(3):185-91. Available from: https://pubmed.ncbi.nlm.nih.gov/9463944/
- Au W, Pathak S, Collie CJ, Hsu TC. Cytogenetic toxicity of gentian violet and crystal violet on mammalian cells in vitro. Mutat Res. 1978;58(2-3):269-76. Available from: https://pubmed.ncbi.nlm.nih.gov/745616/
- Gill PK, Arora DS, Chander M. Biodecolourization of azo and triphenylmethane dyes by Dichomitus squalens and Phlebia spp. J Ind Microbiol Biotechnol. 2002;28(4):201-3. Available from: https://pubmed.ncbi.nlm.nih.gov/11986919/
- Littlefield NA, Blackwell BN, Hewitt CC, Gaylor DW. Chronic toxicity and carcinogenicity studies of gentian violet in mice. Fundam Appl Toxicol. 1985;5(5):902-12. Available from: https://pubmed.ncbi.nlm.nih.gov/4065463/
- Jurevic RJ, Traboulsi RS, Mukherjee PK, Salata RA, Ghannoum MA; Oral HIV/AIDS Research Alliance Mycology Focus group. Identification of gentian violet concentration that does not stain oral mucosa, possesses anti-candidal activity and is well tolerated. Eur J Clin Microbiol Infect Dis. 2011;30(5):629-33. Available from: https://pubmed.ncbi.nlm.nih.gov/21210170/
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8(4):275-83. Available from: https://pubmed.ncbi.nlm.nih.gov/17380161/
- Schuler M, Green DR. Transcription, apoptosis and p53: catch-22. Trends Genet. 2005;21(3):182-7. Available from: https://pubmed.ncbi.nlm.nih.gov/15734577/
- Yamaguchi M, Vikulina T, Weitzmann MN. Gentian violet inhibits MDA-MB-231 human breast cancer cell proliferation, and reverses the stimulation of osteoclastogenesis and suppression of osteoblast activity induced by cancer cells. Oncol Rep. 2015;34(4):2156-62. Available from: https://pubmed.ncbi.nlm.nih.gov/26260090/
- Mukawera E, Chartier S, Williams V, Pagano PJ, Lapointe R, Grandvaux N. Redox-modulating agents target NOX2-dependent IKKε oncogenic kinase expression and proliferation in human breast cancer cell lines. Redox Biol. 2015;6:9-18. Available from: https://pubmed.ncbi.nlm.nih.gov/26177467/
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. Available from: https://pubmed.ncbi.nlm.nih.gov/20457564/
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983 Jun;16(2):109-110. Available from: https://pubmed.ncbi.nlm.nih.gov/6877845/
- Ibrahim KE, Al-Mutary MG, Bakhiet AO, Khan HA. Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules. 2018;23(8):1848. Available from: https://pubmed.ncbi.nlm.nih.gov/30044410/
- Dey P. Tissue Microtomy: Principle and Procedure. In: Basic and Advanced Laboratory Techniques in Histopathology and Cytology. Singapore: Springer Singapore. 2018; 41-50.
- Wluka A, Olszewski WL. Innate and adaptive processes in the spleen. Ann Transplant. 2006;11(4):22-9. PMID: 17715574.
- Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22(4):189-95. Available from: https://pubmed.ncbi.nlm.nih.gov/22019906/
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013 Jan 28;328(2):222-5. Available from: https://pubmed.ncbi.nlm.nih.gov/23085193/
- Mühl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol. 2003 Sep;3(9):1247-55. Available from: https://pubmed.ncbi.nlm.nih.gov/12890422/
- Zhao X, Li R, Huang J, Li J, Hou M, Zhong J. Association of some physiological factors and milk performance in Chinese Holstein. Asian J Anim Vet Adv. 2012;7(12):1356–63. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/20133021289
- Fraile L, Crisci E, Córdoba L, Navarro MA, Osada J, Montoya M. Immunomodulatory properties of beta-sitosterol in pig immune responses. Int Immunopharmacol. 2012;13(3):316-21. Available from: https://pubmed.ncbi.nlm.nih.gov/22595193/
- Chauhan RS. Nutrition, immunity, and livestock health. Indian Cow Sci Econ J. 2010;7(24):2-13.
- Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27-37. Available from: https://pubmed.ncbi.nlm.nih.gov/17426506/
- Bonner MY, Arbiser JL. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell Mol Life Sci. 2012;69(14):2435-42. Available from: https://pubmed.ncbi.nlm.nih.gov/22581366/
- Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005;4(1):3-8. Available from: https://pubmed.ncbi.nlm.nih.gov/15720228/
- Hong KH, Cho ML, Min SY, Shin YJ, Yoo SA, Choi JJ, et al. Effect of interleukin-4 on vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Clin Exp Immunol. 2007;147(3):573-9. Available from: https://pubmed.ncbi.nlm.nih.gov/17302909/
- Li Z, Chen L, Qin Z. Paradoxical roles of IL-4 in tumor immunity. Cell Mol Immunol. 2009 Dec;6(6):415-22. Available from: https://pubmed.ncbi.nlm.nih.gov/20003817/
- Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21(3):339-59. Available from: https://pubmed.ncbi.nlm.nih.gov/10666777/
- Yoshimoto T. The Hunt for the Source of Primary Interleukin-4: How We Discovered That Natural Killer T Cells and Basophils Determine T Helper Type 2 Cell Differentiation in vivo. Front Immunol. 2018;9:716. Available from: https://pubmed.ncbi.nlm.nih.gov/29740428/
- Junttila IS. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front Immunol. 2018;9:888. Available from: https://pubmed.ncbi.nlm.nih.gov/29930549/
- Bao K, Reinhardt RL. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 2015;75(1):25-37. Available from: https://pubmed.ncbi.nlm.nih.gov/26073683/
- Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med. 1993;178(5):1655-63. Available from: https://pubmed.ncbi.nlm.nih.gov/8228812/
- Peleman R, Wu J, Fargeas C, Delespesse G. Recombinant interleukin 4 suppresses the production of interferon gamma by human mononuclear cells. J Exp Med. 1989;170(5):1751-6. Available from: https://pubmed.ncbi.nlm.nih.gov/2530302/
- Lai KN, Leung JC, Li PK, Lui SF. Cytokine production by peripheral blood mononuclear cells in IgA nephropathy. Clin Exp Immunol. 1991;85(2):240-5. Available from: https://pubmed.ncbi.nlm.nih.gov/1907530/
- Morita Y, Yamamura M, Kawashima M, Aita T, Harada S, Okamoto H, et al. Differential in vitro effects of IL-4, IL-10, and IL-13 on proinflammatory cytokine production and fibroblast proliferation in rheumatoid synovium. Rheumatol Int. 2001;20(2):49-54. Available from: https://pubmed.ncbi.nlm.nih.gov/11269532/
- Choi P, Reiser H. IL-4: role in disease and regulation of production. Clin Exp Immunol. 1998;113(3):317-9. Available from: https://pubmed.ncbi.nlm.nih.gov/9737656/
- Legård GE, Pedersen BK. Muscle as an Endocrine Organ. In: Muscle and Exercise Physiology. Elsevier. 2019; 285-307. Available from: https://www.researchgate.net/publication/330053557_Muscle_as_an_Endocrine_Organ
- Deka H, Siddique MA, Ahmed SJ, Mahanta P, Mahanta P. Evaluation of IL-4 and IL-13 Single Nucleotide Polymorphisms and Their Association With Childhood Asthma and Its Severity: A Hospital-Based Case-Control Study. Cureus. 2024;16(4):e57465. Available from: https://pubmed.ncbi.nlm.nih.gov/38699097/